A new machine-learning-based prediction of survival in patients with end-stage liver disease
Comparision against MELD, MELD-Na, MELD 3.0, MELD-Plus7.
Sebastian Gibb1,3,✉, Thomas Berg2, Adam Herber2, Berend Isermann1, and Thorsten Kaiser1,4
2022-12-19
Abstract
Objectives: The shortage of grafts for liver transplantation requires risk stratification and adequate allocation rules. This study aims to improve the model of end-stage liver disease (MELD) score for 90-day mortality prediction with the help of different machine-learning algorithms.
Methods: We retrospectively analyzed the clinical and laboratory data of 654 patients who were recruited during the evaluation process for liver transplantation at University Hospital Leipzig. After comparing 13 different machine-learning algorithms in a nested cross-validation setting and selecting the best performing one we build a new model to predict 90-day mortality in patients with end-stage liver disease.
Results: Penalized regression algorithms yielded the highest prediction performance in our machine-learning algorithm benchmark. In favor of a simpler model we chose the least absolute shrinkage and selection operator (lasso) regression. Beside the classical MELD variables international normalized ratio (INR) and bilirubin, the lasso regression selected cystatin C over creatinine, as well IL-6, total protein, and cholinesterase. The new model offers improved discrimination and calibration over MELD and MELD with sodium (MELD-Na), MELD 3.0, or the MELD-Plus7 risk score.
Conclusion: We provide a new machine-learning-based model of end-stage liver disease that incorporates synthesis and inflammatory markers and may improve the classical MELD score for 90-day survival prediction.
Keywords: end-stage liver disease; IL-6; machine-learning; MELD; risk stratification; survival estimation
1 Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Paul-List-Str. 13-15, D-04103 Leipzig, Germany.
2 Section of Hepatology, Department of Gastroenterology and Rheumatology, University Hospital Leipzig, Liebigstr. 20, D-04103 Leipzig, Germany.
3 Anesthesiology and Intensive Care Medicine, University Hospital Greifswald, Ferdinand-Sauerbruch-Str., D-17475 Greifswald, Germany.
4 Institute of Laboratory Medicine, Microbiology and Clinical Pathobiochemistry, University Hospital OWL, Hospital Lippe, Röntgenstr. 18, D-32756 Detmold, Germany.
✉ Correspondence: Sebastian Gibb <mail@sebastiangibb.de>
Last updated: 2022-12-19
Checks: 7 0
Knit directory:
ampel-leipzig-meld/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210604) was run prior to running the code in the R Markdown file.
Setting a seed ensures that any results that rely on randomness, e.g.
subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 08eace1. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the
analysis have been committed to Git prior to generating the results (you can
use wflow_publish or wflow_git_commit). workflowr only
checks the R Markdown file, but you know if there are other scripts or data
files that it depends on. Below is the status of the Git repository when the
results were generated:
Ignored files:
Ignored: _targets/
Ignored: analysis/article.md
Ignored: container/
Ignored: logs/
Ignored: scripts/R.sh
Untracked files:
Untracked: submission/
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made
to the R Markdown (analysis/article.Rmd) and HTML (docs/article.html)
files. If you’ve configured a remote Git repository (see
?wflow_git_remote), click on the hyperlinks in the table below to
view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 08eace1 | Sebastian Gibb | 2022-12-19 | fix: typo |
| html | 9b17f62 | Sebastian Gibb | 2022-12-19 | chore: rebuild site |
| Rmd | 7ad2405 | Sebastian Gibb | 2022-12-19 | feat: add some paragraphs for variable importance |
| html | 2877c0f | Sebastian Gibb | 2022-12-01 | chore: rebuild site |
| Rmd | e1e7217 | Sebastian Gibb | 2022-12-01 | fix: missing linebreak |
| html | e1e7217 | Sebastian Gibb | 2022-12-01 | fix: missing linebreak |
| html | 88f7b9f | Sebastian Gibb | 2022-12-01 | chore: rebuild site |
| Rmd | b4631f6 | Sebastian Gibb | 2022-12-01 | fix: typos |
| html | 5235082 | Sebastian Gibb | 2022-11-30 | chore: rebuild site |
| Rmd | e1aebf7 | Sebastian Gibb | 2022-11-30 | feat: add MELD 3.0 and adapt to reviewer’s suggestions |
| Rmd | fcbbea2 | Sebastian Gibb | 2022-11-27 | feat: use mortality estimates from VanDerwerken et al. (2021) |
| Rmd | 97b0f1d | Sebastian Gibb | 2022-11-27 | docs: typo |
| html | 1821ea1 | Sebastian Gibb | 2022-08-10 | chore: rebuild site |
| Rmd | 6315aaa | Sebastian Gibb | 2022-08-10 | refactor: drop DS from author list |
| html | 0d43824 | Sebastian Gibb | 2022-08-09 | chore: rebuild site |
| Rmd | bbdb3a2 | Sebastian Gibb | 2022-08-09 | fix: add dash between machine-learning |
| html | afaab45 | Sebastian Gibb | 2022-08-09 | chore: rebuild site |
| Rmd | 288ec2a | Sebastian Gibb | 2022-08-09 | refactor: add TB’s COI |
| html | 1403bda | Sebastian Gibb | 2022-08-09 | chore: rebuild site |
| Rmd | 34c63ff | Sebastian Gibb | 2022-08-09 | fix: add Thorsten’s new affiliation |
| Rmd | 849ca09 | Sebastian Gibb | 2022-08-09 | refactor: add additional co-author |
| Rmd | 84aeda1 | Sebastian Gibb | 2022-08-09 | refactor: integrate Berend’s suggestions |
| Rmd | f766011 | Sebastian Gibb | 2022-08-09 | refactor: change title |
| html | 68ef9aa | Sebastian Gibb | 2022-07-22 | chore: rebuild site |
| Rmd | e6cf8a2 | Sebastian Gibb | 2022-07-22 | refactor: apply proofreading suggestions |
| html | 83e3c7a | Sebastian Gibb | 2022-07-19 | chore: rebuild site |
| Rmd | 4756fb7 | Sebastian Gibb | 2022-07-19 | feat: extend figure captions |
| Rmd | 112a4fe | Sebastian Gibb | 2022-07-19 | fix: labm requires vs. |
| Rmd | 2155e58 | Sebastian Gibb | 2022-07-19 | refactor: create png and pdf just for non-html output |
| Rmd | c775a67 | Sebastian Gibb | 2022-07-19 | refactor: replace DiagrammeR CONSORT diagram by consort |
| Rmd | 2af46fe | Sebastian Gibb | 2022-07-18 | fix: typo |
| html | f86c368 | Sebastian Gibb | 2022-07-18 | chore: rebuild site |
| Rmd | d332cbe | Sebastian Gibb | 2022-07-18 | refactor: labm requires upper case litre |
| Rmd | 4a757df | Sebastian Gibb | 2022-07-18 | refactor: move data and software availability section |
| Rmd | 5435d48 | Sebastian Gibb | 2022-07-18 | refactor: reorder keywords |
| html | 65d58c4 | Sebastian Gibb | 2022-07-18 | chore: rebuild site |
| Rmd | 0ac4a67 | Sebastian Gibb | 2022-07-18 | refactor: restruct headings |
| Rmd | b881188 | Sebastian Gibb | 2022-07-18 | refactor: labm requires down style title |
| Rmd | a0f1d2e | Sebastian Gibb | 2022-07-18 | feat: generate png and pdfs for each plot |
| Rmd | 9d4a890 | Sebastian Gibb | 2022-07-18 | feat: use 1200 dpi as required by labm for word output |
| Rmd | 93871a1 | Sebastian Gibb | 2022-07-18 | feat: add author contributions and competing interests section |
| Rmd | 3cd9643 | Sebastian Gibb | 2022-07-18 | chore: add additional authors |
| Rmd | abae68a | Sebastian Gibb | 2022-07-18 | refactor: drop IQR for median MELD score in limitations |
| Rmd | e81cbe1 | Sebastian Gibb | 2022-07-18 | feat: add vancouver csl |
| Rmd | 057248f | Sebastian Gibb | 2022-07-18 | refactor: replace gaertner2022 by sealock2022 |
| Rmd | 024a0e0 | Sebastian Gibb | 2022-07-17 | refactor: integrate Thorsten’s review comments |
| html | 653f778 | Sebastian Gibb | 2022-07-08 | chore: rebuild site |
| Rmd | e976109 | Sebastian Gibb | 2022-07-08 | feat: add machine learning to keywords |
| Rmd | fab4e55 | Sebastian Gibb | 2022-07-08 | fix: change center to Leipzig |
| html | dcd9153 | Sebastian Gibb | 2022-07-03 | chore: rebuild site |
| Rmd | 048c9bb | Sebastian Gibb | 2022-07-03 | feat: round spearman correlation to 2 digits |
| Rmd | 6c15d1c | Sebastian Gibb | 2022-07-03 | refactor: integrate Bernd’s suggestions |
| Rmd | 464175f | Sebastian Gibb | 2022-07-03 | fix: cystatin c |
| html | 9da49ce | Sebastian Gibb | 2022-06-19 | chore: rebuild site |
| Rmd | 3968729 | Sebastian Gibb | 2022-06-19 | feat: cite ameld with zenodo doi |
| html | fb43d01 | Sebastian Gibb | 2022-06-19 | chore: rebuild site |
| Rmd | abe89ed | Sebastian Gibb | 2022-06-19 | fix: cite zlog with zenodo doi |
| Rmd | a10ff91 | Sebastian Gibb | 2022-06-16 | fix: don’t echo code chunks |
| html | ebe29cf | Sebastian Gibb | 2022-06-16 | chore: rebuild site |
| Rmd | 43a6574 | Sebastian Gibb | 2022-06-16 | feat: further work on the manuscript |
| html | 8035219 | Sebastian Gibb | 2022-06-15 | chore: rebuild site |
| Rmd | 19d9fed | Sebastian Gibb | 2022-06-15 | feat: further work on the manuscript |
| html | d3e9462 | Sebastian Gibb | 2022-06-06 | chore: rebuild site |
| Rmd | 3181fbe | Sebastian Gibb | 2022-06-06 | refactor: suppress warning for table1 |
| html | b20484a | Sebastian Gibb | 2022-06-06 | chore: rebuild site |
| Rmd | 01f934b | Sebastian Gibb | 2022-06-06 | feat: add first discussion draft |
| Rmd | 13ac21e | Sebastian Gibb | 2022-06-06 | feat: add docx export |
| html | 983ec69 | Sebastian Gibb | 2022-03-17 | chore: rebuild site |
| Rmd | 85ff071 | Sebastian Gibb | 2022-03-15 | fix: co2eq calculation |
| Rmd | 839f920 | Sebastian Gibb | 2022-03-11 | fix: cite timeROC package |
| Rmd | 4a51c7d | Sebastian Gibb | 2022-03-11 | feat: add co2eq estimation |
| Rmd | c5d6267 | Sebastian Gibb | 2022-03-10 | feat: describe model development in methods section |
| Rmd | 74483a5 | Sebastian Gibb | 2022-02-10 | chore: remove comments |
| Rmd | 09c3fab | Sebastian Gibb | 2022-02-10 | refactor: switch to grid search |
| html | 373e7d8 | Sebastian Gibb | 2021-10-20 | chore: rebuild site |
| Rmd | 8b3ba9c | Sebastian Gibb | 2021-10-19 | feat: cite gtsummary |
| Rmd | 28ad932 | Sebastian Gibb | 2021-10-19 | feat: describe zlog in more detail |
| html | df8964f | Sebastian Gibb | 2021-10-15 | chore: rebuild site |
| Rmd | 2697ead | Sebastian Gibb | 2021-10-15 | fix: remove missing cross reference |
| Rmd | eeca976 | Sebastian Gibb | 2021-10-15 | feat: add ml method part |
| html | c66640c | Sebastian Gibb | 2021-09-14 | feat: first nnet tests |
| html | afa48d9 | Sebastian Gibb | 2021-08-07 | chore: rebuild site |
| Rmd | d32f7b3 | Sebastian Gibb | 2021-08-07 | fix: gtsummary compact theme |
| Rmd | ebf878b | Sebastian Gibb | 2021-08-07 | fix: observed vs expected mortality table |
| Rmd | 4ec3d40 | Sebastian Gibb | 2021-08-04 | feat: add table 1 and table observed vs expected mortality |
| Rmd | e41dd8c | Sebastian Gibb | 2021-08-03 | feat: add flowchart and discussion notes |
| Rmd | 523c286 | Sebastian Gibb | 2021-08-02 | refactor: use bookdown::html_document2 instead of workflowr::wflow_html to allow crossreferences |
| html | 3aab3e1 | Sebastian Gibb | 2021-08-01 | chore: rebuild site |
| Rmd | 91bfbd2 | Sebastian Gibb | 2021-08-01 | feat: add first article draft with pandoc filters |
1 Introduction
Liver cirrhosis is the terminal result of the fibrotic remodeling of liver tissue due to chronic damage. This end-stage of liver disease is usually irreversible, and the only available therapy is liver transplantation. However, the shortage of grafts for transplantation from deceased donors requires risk stratification and precise and fair allocation rules. The allocation of liver transplantation in most countries is based on disease severity determined by the model of end-stage liver disease (MELD) (Malinchoc et al. 2000; Wiesner et al. 2003; Organ Procurement and Transplantation Network 2021). The MELD score estimates patients’ 3-month mortality risk based on laboratory results, namely bilirubin, creatinine, and the international normalized ratio (INR). The MELD score has been extended by the sodium level (MELD-Na score) because this was found to be an important additional risk factor in liver cirrhosis (Kim et al. 2008; Organ Procurement and Transplantation Network 2021). Recently MELD 3.0 was introduced adding a factor for female sex, albumin and interactions between bilirubin and sodium and between albumin and creatinine (Kim et al. 2021). The MELD was initially developed to predict the survival of patients undergoing transjugular intrahepatic portosystemic shunts. It was subsequently revalidated to predict mortality risk in patients awaiting a liver transplantation. Although the MELD score should be an objective allocation score, creatinine and INR are highly dependent on the laboratory methods used (Trotter et al. 2004; Cholongitas et al. 2007). In addition women are disadvantaged in MELD-Na (Sealock et al. 2022). Patients with identical disease states can have very different MELD scores and thus receive different priority levels on the liver transplantation waiting list. Furthermore, for acute-on-chronic liver failure, for example, the MELD score often underestimates the mortality risk (Hernaez et al. 2020).
There have been some attempts to use the data extracted from more than 300,000 electronic medical records from two hospitals in the United States to improve the MELD score (Kartoun et al. 2017). The derived MELD-Plus7 and MELD-Plus9 risk scores add albumin, white blood cell count, total cholesterol, age, and length of stay to the MELD-Na variables. Despite their published prediction improvement, the MELD-Plus scores are not yet used for transplant allocation.
As shown by the MELD-Plus risk and MELD 3.0 scores, better predictive scores often need more variables and are more complicated. To reduce the risk of overlooking or incorrectly calculating and interpreting the results, clinical decision support systems may be used and could improve patient safety. The research project on digital laboratory medicine (AMPEL) develops a clinical decision support system based on laboratory diagnostics that should support clinical practitioners in interpreting laboratory results and taking the necessary medical interventions (Eckelt et al. 2020).
This study aims to find clinical and laboratory values that improve on the risk stratification for liver transplantation of the classical MELD, MELD-Na, MELD 3.0, and MELD-Plus scores and that might be implemented as part of the AMPEL clinical decision support system.
2 Material and methods
2.1 Study population
In a retrospective cohort study we followed 778 consecutive patients, who were recruited during the evaluation process for liver transplantation at University Hospital Leipzig from November 2012 to June 2015. For each patient, we recorded 44 variables, including age, sex, etiology of liver disease, complications as listed in Table 3.1, and 25 laboratory measurements.
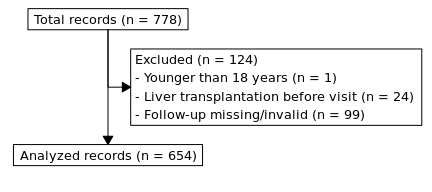
Figure 2.1: Flowchart showing inclusion and exclusion of records.
We excluded 124 patients from our analysis (Figure 2.1). One was younger than 18 years, 24 had had a liver transplant before and in 99 cases the follow-up data were missing or invalid.
The Ethics Committee at the Leipzig University Faculty of Medicine approved the retrospective usage of the data for our study (reference number: 039/14ff).
2.2 MELD scores
MELD and MELD-Na were calculated as described in Organ Procurement and Transplantation Network (2021) using the following formulas: \(MELD = 10 * (0.957 \ln(creatinine [mg/dL]) + 0.378 \ln(bilirubin [mg/dL]) + 1.120 * \ln(INR) + 0.643)\), Creatinine, bilirubin and INR values lower than 1.0 mg/dL were set to 1.0 mg/dL. The maximum allowed creatinine was 4.0 mg/dL. If the patients received dialysis, creatinine was set to 4.0 mg/dL. For MELD-Na, MELD was calculated as above and recalculated if greater than 11 using: \(MELD\text{-}Na = MELD_i + 1.32 * (137 - Na [mmol/L]) - (0.033 * MELD_i * (137 - Na [mmol/L]))\)
Serum sodium values lower than 125 mmol/L or higher than 137 mmol/L were set to 125 mmol/L and 137 mmol/L, respectively.
MELD 3.0 was calculated as described in kim2021:
\[ \begin{aligned} MELD3 = 1.33 (if female) &+ \\ 4.56 &* \ln(bilirubin [mg/dL]) + \\ 0.82 &* (137 - Na [mmol/L]) - 0.24 * (137 - Na [mmol/L]) * \ln(bilirubin [mg/dL]) + \\ 9.09 &* \ln(INR) + \\ 11.14 &* \ln(creatinine [mg/dL]) + \\ 1.85 &* (3.5 - albumin [g/dL]) - 1.83 * (3.5 - albumin [g/dL]) * \ln(creatinine [mg/dL]) + 6 \end{aligned} \] In contrast to MELD-Na the maximum allowed creatinine was set to 3.0 mg/dL.
The MELD-Plus7 risk score was calculated as described in Kartoun et al. (2017): $$ \[\begin{aligned} L = 8.53499496 &+ \\ 2.59679650 &* \log_{10}(1 + creatinine [mg/dL]) + \\ 2.06503238 &* \log_{10}(1 + bilirubin [mg/dL]) + \\ 2.99724802 &* \log_{10}(1 + INR) - \\ 6.47834101 &* \log_{10}(1 + sodium [mmol/L]) - \\ 6.34990436 &* \log_{10}(1 + albumin [g/dL]) + \\ 1.92811726 &* \log_{10}(1 + wbc [th/cumm]) + \\ 0.04070442 &* age [years] \end{aligned}\]$
\[MELD\text{-}Plus = \frac{\exp(L)}{1 + \exp(L)}\]
The MELD-Plus9 score was ignored because the length of stay information was not available for our cohort, and the model itself was not superior to MELD-Plus7 in its original publication (Kartoun et al. 2017).
2.3 Data processing and machine-learning
All data processing, statistical, and machine-learning analyses were performed using R version 4.2.0 (R Core Team 2022). Prior to the analyses, all laboratory values were zlog transformed to approximate a normal distribution as described in Hoffmann et al. (2017) and implemented in Gibb (2021).
\[ z = (\log(x) - \frac{\log(LL) + \log(UL)}{2}) * \frac{3.92}{\log(UL) - \log(LL)} \]
where \(LL\) and \(UL\) are the lower and upper age-, and sex-related reference limits of the corresponding laboratory diagnostic, respectively. Missing laboratory measurements were replaced with a zlog value of zero. In a similar manner, where information about complications or comorbidities was missing, we treated them as not present.
We compared 13 different statistical and machine-learning algorithms using the mlr3 framework (Lang et al. 2019, 2022; Sonabend et al. 2021; Sonabend, Kiraly, and Lang 2022). The algorithms used were designed for analysis of survival data: Cox proportional hazards regression model (Cox 1972; Terry M. Therneau and Patricia M. Grambsch 2000; Therneau 2022); penalized regressions, namely, two different implementations of the lasso (least absolute shrinkage and selection operator) regression (Tibshirani 1997; Simon et al. 2011; Friedman et al. 2022; J. J. Goeman 2010; J. Goeman et al. 2022), two different implementations of the ridge regression (Simon et al. 2011; Friedman et al. 2022; J. J. Goeman 2010; J. Goeman et al. 2022), and the elastic net regression (Simon et al. 2011; Friedman et al. 2022); two different random forest implementations (Wright and Ziegler 2017; Wright, Wager, and Probst 2021; H. Ishwaran et al. 2008; Hemant Ishwaran and Kogalur 2020); extreme gradient boosting (T. Chen and Guestrin 2016; Tianqi Chen et al. 2022); two different support vector machines (Belle et al. 2011; Fouodo 2018); and two neural networks (Katzman et al. 2018; Kvamme, Borgan, and Scheel 2019; Sonabend 2022).
We used nested resampling to avoid feature- and model-selection-bias to obtain reliable metrics to choose the best model. We thus applied a 5-fold inner and 3-fold outer cross-validation that was repeated 10 times. We searched the hyperparameter space using a grid search with a resolution of 10. The models were evaluated using the concordance index, which is a measure of rank correlation between predicted and observed events (Harrell 1982). Subsequently, we selected the model with the highest median concordance index. If multiple models were comparable, we chose the simpler (more penalized) one.
After selecting the final model, we trained and validated it as described in Harrell, Lee, and Mark (1996). Briefly, we developed our model using a 3-fold cross-validation that was repeated 10 times and finally predicted survival at 90 days. The predictions were sorted and grouped into equal-sized intervals so that there were 50 patients in each interval. For each group, the mean survival probability and the difference to the Kaplan-Meier estimate was calculated. The same procedure was repeated for 200 bootstrap samples. Subsequently, the differences between predicted survival and Kaplan-Meier estimates of the bootstrap samples were averaged and added to the original model difference to obtain a bias-corrected estimate between predicted and observed survival.
The final model was compared against the MELD, MELD-Na, MELD 3.0 and MELD-Plus7 scores using the area under the time-dependent receiver operating characteristic curve (AUROC) based on the nonparametric inverse probability of censoring weighting estimator (IPCW) Paul Blanche (2019). Testing was applied as described in P. Blanche, Dartigues, and Jacqmin-Gadda (2013). A \(p < 0.05\) was considered a statistically significant difference.
Further we determined the variable importance by counting how often the variable was chosen during the feature selection in the bootstrap process described above and by the logrank value returned by one random forest implementation (Wright, Wager, and Probst 2021).
Summary tables were built using the gtsummary package (Sjoberg et al. 2021, 2022).
3 Results
3.1 Baseline characteristics
| Characteristic | Female, N = 240 | Male, N = 414 | Overall, N = 654 |
|---|---|---|---|
| General | |||
| Follow-up time [days] | 181 (17, 411) | 198 (54, 378) | 191 (38, 384) |
| Age | 56 (51, 64) | 58 (52, 63) | 58 (52, 64) |
| LTx | 20 (8.3%) | 40 (9.7%) | 60 (9.2%) |
| Laboratory measurements | |||
| Total bilirubin [µmol/L] | 21 (10, 49) | 26 (14, 51) | 24 (13, 50) |
| Cystatin C [mg/L] | 1.31 (1.04, 1.80) | 1.32 (1.08, 1.76) | 1.32 (1.07, 1.79) |
| (Missing) | 2 | 5 | 7 |
| Creatinine [µmol/L] | 73 (58, 91) | 85 (71, 110) | 80 (65, 104) |
| INR | 1.20 (1.06, 1.50) | 1.22 (1.10, 1.44) | 1.21 (1.09, 1.46) |
| (Missing) | 1 | 1 | 2 |
| Sodium [mmol/L] | 138.8 (135.4, 141.0) | 138.0 (135.0, 140.1) | 138.1 (135.2, 140.4) |
| (Missing) | 5 | 4 | 9 |
| WBC [exp 9/L] | 6.7 (4.7, 8.8) | 6.2 (4.6, 7.8) | 6.3 (4.7, 8.3) |
| (Missing) | 10 | 13 | 23 |
| IL-6 [pg/mL] | 10 (5, 36) | 14 (6, 47) | 13 (6, 42) |
| (Missing) | 27 | 29 | 56 |
| Albumin [g/L] | 41 (35, 46) | 38 (33, 43) | 39 (34, 45) |
| (Missing) | 2 | 4 | 6 |
| Total protein [g/L] | 71 (63, 76) | 71 (65, 76) | 71 (65, 76) |
| (Missing) | 2 | 4 | 6 |
| Cholesterine [mmol/L] | 4.64 (3.41, 5.56) | 4.28 (3.34, 5.15) | 4.40 (3.34, 5.31) |
| ALAT [µkat/L] | 0.29 (0.21, 0.42) | 0.36 (0.23, 0.56) | 0.33 (0.22, 0.48) |
| (Missing) | 2 | 8 | 10 |
| ASAT [µkat/L] | 0.68 (0.49, 1.05) | 0.81 (0.61, 1.23) | 0.77 (0.55, 1.16) |
| (Missing) | 3 | 5 | 8 |
| MELD | |||
| MELD score | 10 (7, 18) | 12 (9, 17) | 11 (8, 17) |
| (Missing) | 2 | 1 | 3 |
| MELD-Na score | 10 (7, 19) | 12 (9, 20) | 11 (8, 20) |
| (Missing) | 5 | 4 | 9 |
| MELD Category | |||
| [6,9] | 111 (47%) | 146 (36%) | 257 (40%) |
| [10,20) | 71 (30%) | 163 (40%) | 234 (36%) |
| [20,30) | 28 (12%) | 77 (19%) | 105 (16%) |
| [30,40) | 21 (8.9%) | 17 (4.1%) | 38 (5.9%) |
| [40,52) | 4 (1.7%) | 7 (1.7%) | 11 (1.7%) |
| (Missing) | 5 | 4 | 9 |
| MELD 3.0 score | 12 (8, 20) | 12 (9, 19) | 12 (8, 19) |
| (Missing) | 8 | 8 | 16 |
| Entities | |||
| Cirrhosis | 203 (85%) | 391 (94%) | 594 (91%) |
| (Missing) | 1 | 0 | 1 |
| ALF | 6 (2.5%) | 2 (0.5%) | 8 (1.2%) |
| CLI | 30 (12%) | 21 (5.1%) | 51 (7.8%) |
| Etiology (more than 1 per patient possible) | |||
| Ethyltoxic | 114 (48%) | 297 (72%) | 411 (63%) |
| HBV | 4 (1.7%) | 16 (3.9%) | 20 (3.1%) |
| HCV | 17 (7.1%) | 28 (6.8%) | 45 (6.9%) |
| AIH | 22 (9.2%) | 9 (2.2%) | 31 (4.7%) |
| PBC | 17 (7.1%) | 0 (0%) | 17 (2.6%) |
| PSC | 5 (2.1%) | 11 (2.7%) | 16 (2.4%) |
| NASH | 17 (7.1%) | 31 (7.5%) | 48 (7.3%) |
| Cryptogenic | 36 (15%) | 32 (7.7%) | 68 (10%) |
| Complications (more than 1 per patient possible) | |||
| Dialysis | 14 (5.9%) | 19 (4.6%) | 33 (5.1%) |
| (Missing) | 1 | 1 | 2 |
| GIB | 58 (24%) | 104 (25%) | 162 (25%) |
| HCC | 18 (7.5%) | 103 (25%) | 121 (19%) |
| SBP | 31 (13%) | 60 (15%) | 91 (14%) |
| (Missing) | 0 | 1 | 1 |
| Mortality | |||
| Within 7 days | 16 (7.2%) | 18 (4.5%) | 34 (5.5%) |
| Within 30 days | 25 (12%) | 31 (8.1%) | 56 (9.4%) |
| Within 90 days | 32 (16%) | 49 (13%) | 81 (14%) |
| Within 365 days | 39 (20%) | 61 (18%) | 100 (18%) |
In this study, we analyzed a cohort of 654 patients evaluated for liver transplantation (Tab. 3.1). Of these, 414 (63%) were men. The median (IQR) follow-up times was 191 (38, 384) days. Within the follow-up time, 60 (9.2%) patients received a liver transplant and were censored for the analysis beyond this time point. The median (IQR) age of the cohort was 58 (52, 64) years.
Most of the patients presented with cirrhosis (594 (91%)). The remaining patients had acute liver failure (8 (1.2%)) or chronic liver insufficiency (51 (7.8%)). At evaluation time, the median (IQR) MELD, MELD-Na and MELD 3.0 scores were 11 (8, 17), 11 (8, 20), and 12 (8, 19), respectively. Within 90 days, 81 (14%) patients had died. In nearly two thirds of all patients (411 (63%)), alcohol was the main cause of their end-stage liver disease. The remaining patients had viral infections - hepatitis B (20 (3.1%)) or C (45 (6.9%)) - autoimmune autoimmune (31 (4.7%)), primary sclerosing cirrhosis (16 (2.4%)), primary biliary cirrhosis (17 (2.6%)), unknown causes (non-alcoholic steatohepatitis (48 (7.3%)), or cryptogenic hepatitis (68 (10%))). The most common complications among all patients were gastrointestinal bleedings in 162 (25%) patients, followed by hepatocellular carcinoma (HCC) in 121 (19%) and spontaneous bacterial peritonitis (SBP) in 91 (14%) patients. Just 33 (5.1%) patients required renal dialysis.
3.2 MELD scores
We divided our cohort into 5 MELD-Na-score risk categories, with 257, 234, 105, 38, and 11 patients, respectively. As shown in Table 3.2, our observed 90-day mortality was 1.2 to 2.2 times higher than the predicted one (VanDerwerken et al. 2021). Only in the group with the lowest MELD-Na scores was the mortality much lower.
| MELD-Na category | Observed deaths (n) | Expected deaths (n) | Standardized mortality ratio (SMR) | Observed mortality (%) | Expected mortality (%) |
|---|---|---|---|---|---|
| [6,9] | 1 | 5.8 | 0.2 | 0.5 | 2.8 |
| [10,20) | 9 | 6.9 | 1.3 | 4.5 | 3.7 |
| [20,30) | 37 | 17.0 | 2.2 | 40.9 | 22.0 |
| [30,40) | 23 | 16.2 | 1.4 | 87.5 | 64.6 |
| [40,52) | 6 | 5.1 | 1.2 | 100.0 | 84.4 |
3.3 AMPEL MELD
The penalized regression and random forest models resulted in a similar high median performance in the benchmark process. In favour of simpler models, we chose a lasso-based approach for the final model development. The classical Cox model, the data and computational intense algorithms extreme gradient boosting, the support vector machines and the neural networks yielded a worse performance.
| Coefficient | |
|---|---|
| IL-6 | 0.193 |
| Total protein | -0.081 |
| INR | 0.067 |
| Cholinesterase | -0.037 |
| Cystatin C | 0.026 |
| Direct bilirubin | 0.010 |
The resulting AMPEL model of end-stage liver disease (AMELD) uses 6 predictors. Besides the classical MELD variables INR and (direct) bilirubin, the lasso regression selected cystatin C over creatinine, as well as IL-6, total protein, and cholinesterase.
| t [days] | 2 | 7 | 30 | 90 | 180 | 365 |
|---|---|---|---|---|---|---|
| S0 (t) | 0.9967 | 0.9912 | 0.9816 | 0.9661 | 0.9546 | 0.9489 |
The 90-day survival estimate of a given patient can be calculated as shown in (3.1).
\[\begin{align} S &= S_{0,90}^{\exp{LP}} \\ &= 0.9661 ^{\exp{( 0.1929 * zlog(\text{IL-6})-0.0805 * zlog(\text{Total protein})+0.0667 * zlog(\text{INR})-0.0368 * zlog(\text{Cholinesterase})+0.026 * zlog(\text{Cystatin C})+0.0098 * zlog(\text{Direct bilirubin}) )}} \tag{3.1} \end{align} \]
where \(S_{0}\) is the baseline survival estimate from 3.4 and \(LP\) is the linear predictor of the coefficients in 3.3.
3.4 Model comparison
![Receiver operating characteristic (ROC) curve. Area under the time-dependent ROC curve (AUC) based on the nonparametric inverse probability of censoring weighting estimate (IPCW) for AMELD, MELD, MELD-Na, MELD 3.0, MELD-Plus7, as described in [@blanche2013].](figure/article.Rmd/roc-1.png)
Figure 3.1: Receiver operating characteristic (ROC) curve. Area under the time-dependent ROC curve (AUC) based on the nonparametric inverse probability of censoring weighting estimate (IPCW) for AMELD, MELD, MELD-Na, MELD 3.0, MELD-Plus7, as described in (P. Blanche, Dartigues, and Jacqmin-Gadda 2013).
| t=2 | t=7 | t=30 | t=90 | t=180 | t=365 | |
|---|---|---|---|---|---|---|
| Non-adjusted \(p\)-values | 0.27 | 0.15 | 0.33 | 0.03 | 0.02 | 0.33 |
| Adjusted \(p\)-values | 0.75 | 0.52 | 0.84 | 0.15 | 0.08 | 0.84 |
AMELD offers the best AUROC 0.961 (95% CI, 0.942-0.980) of all competitors. However, after correction for multiple testing, the difference against the second-best model, MELD 3.0 (AUROC 0.936 (95% CI, 0.910-0.963)), is not statistical significant (\(p = 0.15\)) (see Table 3.5 for complete comparison).
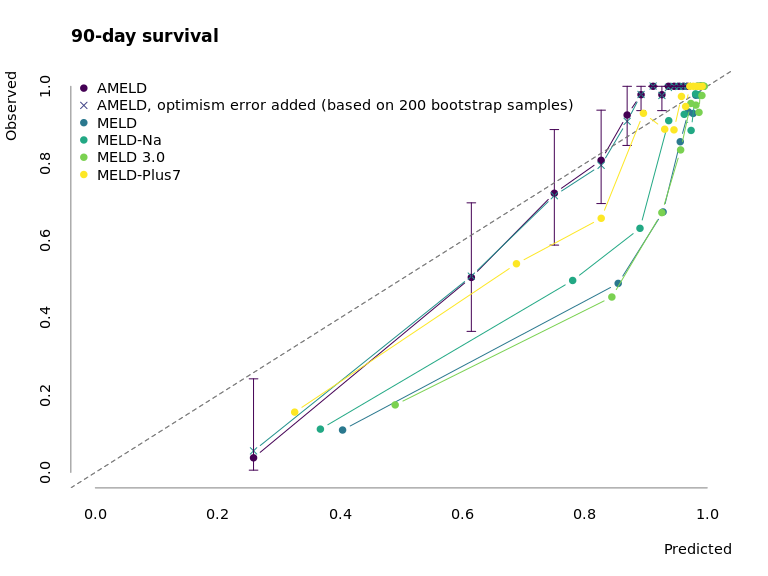
Figure 3.2: Calibration of AMELD in comparison to MELD, MELD-Na, MELD 3.0 and MELD-Plus7. The points mark the mean predicted survival of 50 patients per interval against their observed mean survival. The error bars correspond to the 95% confidence interval of the survival estimate.
Fig. 3.2 depicts the calibration curve for AMELD and the competing MELD scores. While the prediction for low-risk patients is similar to other scores, AMELD predicts the medium-risk patients more accurately.
3.5 Variable importance
The AMELD selected features IL-6, total protein, INR, cholinesterase, cystatin C, and direct bilirubin are the 6 highest ranked features in the bootstrap process. In the random forest logrank they are among the 12 highest ranked features out of 42 features. Sodium and albumin introduced by MELD-Na and MELD 3.0 are on position 18 and 12 for the bootstrap and 20 and 5 for the random forest variable importance ranking, respectively (see ??, ?? for the complete rankings).
4 Discussion
In this retrospective analysis of 654 consecutive patients who were recruited during the evaluation process for liver transplantation at University Hospital Leipzig, we developed a new model to predict 90-day survival probability. Our new model, AMELD, incorporates synthesis and inflammatory markers and may improve the prediction over previous published MELD scores.
Although the Organ Procurement and Transplantation Network recommends the use of MELD-Na over MELD, the sodium does not add any information to the score. Neither in the bootstrap selection nor in the variable importance of a random forest model does the sodium gain any important weight (in bootstrap selection rank 18 of 42 and in a random forest variable importance rank 20 of 42, see ??, ??). In addition, sodium levels can be affected by diuretic therapy, and the discrimination performance of MELD-Na has already been shown by Kartoun et al. (2017) to be insignificantly different from the classical MELD. Consequently it is not a component of our model.
The albumin included in MELD 3.0 is part of the total protein that has throughout higher rankings in both variable importance measurements (12 vs. 2 in the bootstrap selection and 5 vs. 1 in the random forest variable importance, see ??, ??). Using total protein instead of albumin as synthesis marker offers more information about additional globulins and especially coagulation factors that are incompletely measured by INR.
It has been shown that women receive median lower scores in MELD-Na and therefore are less likely to receive an urgent listing for liver transplantation (Sealock et al. 2022). MELD 3.0 tries to circumvent this by adding 1.33 to the score for femal sex. To address sexual differences in laboratory measurements we applied the zlog transformation (Hoffmann et al. 2017) in our study. This transformation also yields comparable laboratory measurements across different clinical laboratories and methods, as well as reducing the influence of age- and sex-related differences, which could improve the objectivity of the allocation process. The already nearly normal scaled zlog-transformed laboratory measurements allow us to avoid the sample-dependent scaling prior to model development. Both should increase the external applicability of our approach.
Our model, AMELD, uses 6 predictors. Three of these - INR, (direct) bilirubin, and cystatin C - are identical or similar to the laboratory measurements utilized in the classical MELD. The direct bilirubin selected by our approach and the total bilirubin in MELD are highly correlated (Spearman correlation: 0.96) and therefore interchangeable.
In our feature selection process, cystatin C was chosen instead of creatinine. This is in line with recent studies that show a better prediction of mortality for cystatin C in unselected and critically ill patients (Helmersson-Karlqvist, Ärnlöv, and Larsson 2016; Helmersson-Karlqvist et al. 2021).
Furthermore AMELD, extends MELD by the additional synthesis parameters total protein and cholinesterase, and most importantly the inflammatory marker IL-6.
The latter is an example where results from machine-learning can aid in finding clinically meaningful patterns and guide further research. Systemic inflammatory response syndrome is an independent risk factor for poor outcomes in patients with cirrhosis (Thabut et al. 2007). However, neither MELD, MELD-Na, nor MELD 3.0 took inflammation into account. By contrast, IL-6 is the most important variable in our model. Even if IL-6 is excluded, the algorithms select CRP as one of the most important variables, which highlights the important role of inflammation in the prognosis of patients with end-stage liver disease. We and other researchers have already shown that adding inflammatory markers, namely IL-6 or CRP, to the classical MELD score increases the accuracy of the 90-day mortality prediction (Remmler et al. 2017; Cervoni et al. 2016).
Besides laboratory measurements, we investigated 17 other items of clinical data, but none were chosen in our model-selection process. Kartoun et al. (2017) also used the lasso approach and did not select any clinical data. In retrospective analyses in particular, the clinical data are often not detailed enough to provide valuable information.
In our benchmark of 13 different machine-learning algorithms we have seen that the more complex algorithms, such as boosting, support vector machines, and neural networks, perform worse than simpler ones. This may be due to additive effects that favor regression models or due to our relatively small sample size. It has been shown that in machine-learning more than 200 events per predictor variable are required to achieve good prediction performance (Ploeg, Austin, and Steyerberg 2014).
4.1 Limitations
Our study has several limitations. Principally, it is a single-center, retrospective analysis lacking an external validation cohort. The data were collected during the evaluation process for liver transplantation at University Hospital Leipzig. There was no controlled follow-up process. We excluded all patients who had already undergone liver transplantation, which could artificially lower the disease severity of the cohort. Furthermore, most of our patients suffered from ethyltoxic liver cirrhosis (63%), which may limited the transfer to other etiologies.
With a median MELD-Na score of 11, our cohort was healthier than those in previous studies that reported a score of 14-18.5 (Kim et al. 2008; Kartoun et al. 2017; VanDerwerken et al. 2021). However, the 90-day mortality rate of 81 (14%) was comparable to the mortality rates seen in earlier studies ranging from 6% to 30% (Malinchoc et al. 2000; Kim et al. 2008; Kartoun et al. 2017). Although our cohort was healthier, with a medium mortality rate, the classical MELD score underestimates the observed mortality by 1.2 to 2.2 times. Unfortunately, our sample size was low in the high-risk patient category, resulting in a poor but better than MELD-based prediction performance by our AMELD for very ill patients.
5 Conclusion
In conclusion, we have developed a new model to predict the 90-day survival of patients during the evaluation process for liver transplantation. Our model, AMELD, extends the classical MELD predictors INR, (direct) bilirubin, and cystatin C (instead of creatinine) by using the synthesis parameters total protein and cholinesterase and, most importantly, the inflammatory marker IL-6. Using these six laboratory measurements, AMELD may improve the prediction over previous published MELD scores. For wider adoption, a prospective multi-center study for model recalibration and validation is needed.
5.1 Acknowledgments
We thank the whole AMPEL team for their support, Stefan Kemnitz for his technical support regarding the use of the high-performance computing (HPC) cluster at the University of Greifswald and Bernd Klaus for helpful discussions about survival analysis and proofreading the manuscript.
The calculations were conducted using the HPC cluster at University of Greifswald. In 2018, the average carbon efficiency of the German power grid was 0.471 kgCO2eq/kWh (Icha, Lauf, and Kuhs 2021). A single run of the complete calculation pipeline took roughly 96 hours of computation on an Intel(R) Xeon(R) E5-2650 v4 and Intel(R) Xeon(R) Gold 6240 CPU (thermal design power: 105 and 150 W). The total estimated emissions were at least 6.77 kgCO2eq (ignoring development, test runs, etc.). This is equivalent to 44 km driven by an average car (Allekotte et al. 2021).
5.2 Research funding
The study was supported by the AMPEL project (www.ampel.care). This project is co-financed through public funds according to the budget decided by the Saxon State Parliament under RL eHealthSax 2017/18 grant number 100331796. The funder provided support in the form of salaries for author SG but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
5.4 Competing interests
TB recieved grants or research support from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan, Sequana Medical. TB received honoraria or consultation fees/advisory board from Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, Falk Foundation, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi TB participated in a company sponsored speaker’s bureau for Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX, and SOBI. All other authors state no conflict of interest.
5.5 Informed consent
Informed consent was obtained from all individuals included in this study.
5.6 Ethical approval
Research involving human subjects complied with all relevant national regulations and institutional policies, as well as the tenets of the Helsinki Declaration (as revised in 2013), and was approved by the Ethics Committee at the Leipzig University Faculty of Medicine (reference number: 039/14ff).
5.7 Data and software availability
The complete code and reproducible analysis can be found at
https://ampel-leipzig.github.io/ampel-leipzig-meld/.
In addition, all data are available in the ameld R package (Gibb 2022) at
https://github.com/ampel-leipzig/ameld
for further investigation.
6 References
sessionInfo()R version 4.2.0 (2022-04-22)
Platform: x86_64-unknown-linux-gnu (64-bit)
Matrix products: default
BLAS/LAPACK: /gnu/store/ras6dprsw3wm3swk23jjp8ww5dwxj333-openblas-0.3.18/lib/libopenblasp-r0.3.18.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] grid stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] gtsummary_1.6.0 consort_1.0.1 timeROC_0.4 ameld_0.0.31
[5] survival_3.3-1 glmnet_4.1-4 Matrix_1.4-1 viridis_0.6.2
[9] viridisLite_0.4.0 mlr3viz_0.5.9 ggplot2_3.3.6 targets_0.12.1
loaded via a namespace (and not attached):
[1] fs_1.5.2 bbotk_0.5.3 rprojroot_2.0.3
[4] mlr3pipelines_0.4.1 mlr3proba_0.4.11 numDeriv_2016.8-1.1
[7] tools_4.2.0 backports_1.4.1 bslib_0.3.1
[10] utf8_1.2.2 R6_2.5.1 mlr3_0.13.3
[13] colorspace_2.0-3 withr_2.5.0 mlr3misc_0.10.0
[16] tidyselect_1.1.2 gridExtra_2.3 processx_3.5.3
[19] compiler_4.2.0 git2r_0.30.1 cli_3.3.0
[22] ooplah_0.2.0 gt_0.6.0 lgr_0.4.3
[25] bookdown_0.26 sass_0.4.1 scales_1.2.0
[28] checkmate_2.1.0 mvtnorm_1.1-3 pec_2022.05.04
[31] callr_3.7.0 palmerpenguins_0.1.0 commonmark_1.8.0
[34] mlr3tuning_0.13.1 stringr_1.4.0 digest_0.6.29
[37] mlr3extralearners_0.5.37 rmarkdown_2.14 param6_0.2.4
[40] paradox_0.9.0 set6_0.2.4 pkgconfig_2.0.3
[43] htmltools_0.5.2 parallelly_1.31.1 highr_0.9
[46] fastmap_1.1.0 rlang_1.0.2 shape_1.4.6
[49] jquerylib_0.1.4 generics_0.1.2 jsonlite_1.8.0
[52] dplyr_1.0.9 magrittr_2.0.3 Rcpp_1.0.8.3
[55] munsell_0.5.0 fansi_1.0.3 lifecycle_1.0.1
[58] stringi_1.7.6 whisker_0.4 yaml_2.3.5
[61] parallel_4.2.0 dictionar6_0.1.3 listenv_0.8.0
[64] promises_1.2.0.1 crayon_1.5.1 lattice_0.20-45
[67] splines_4.2.0 knitr_1.39 ps_1.7.0
[70] pillar_1.7.0 timereg_2.0.2 igraph_1.3.1
[73] uuid_1.1-0 base64url_1.4 future.apply_1.9.0
[76] codetools_0.2-18 glue_1.6.2 evaluate_0.15
[79] broom.helpers_1.7.0 data.table_1.14.2 vctrs_0.4.1
[82] httpuv_1.6.5 foreach_1.5.2 distr6_1.6.9
[85] tidyr_1.2.0 gtable_0.3.0 purrr_0.3.4
[88] future_1.26.1 xfun_0.31 prodlim_2019.11.13
[91] later_1.3.0 tibble_3.1.7 iterators_1.0.14
[94] workflowr_1.7.0 lava_1.6.10 globals_0.15.0
[97] ellipsis_0.3.2